When browsing the official Amazon forum, the editor noticed that the Amazon US website released new regulations on submitting dietary supplement compliance documents on January 11th.
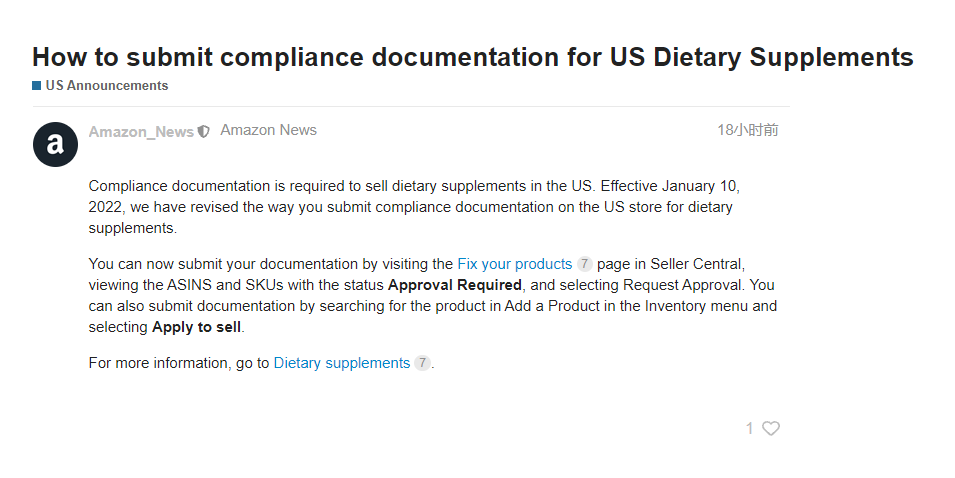
Amazon stated in the announcement: "Selling dietary supplements in the United States requires submission of compliance documents. Starting from January 10, 2022, we have modified the way you submit dietary supplement compliance documents in US stores
Dietary supplement category sellers can view the ASINS and SKU Then select Request Approval. You can also submit documents by searching for products in the "Add Products" section of the "Inventory" menu and selecting "Apply for Sale".
Affected by policies, a large number of sellers in this category have reported that their product links have been deleted recently. Seller "sell4more" stated that Amazon has removed a large number of dietary supplement products, including some of its best-selling products on Amazon. Moreover, 'sell4more' indicates that it has not received any notification regarding the need for compliance documents for this category of products.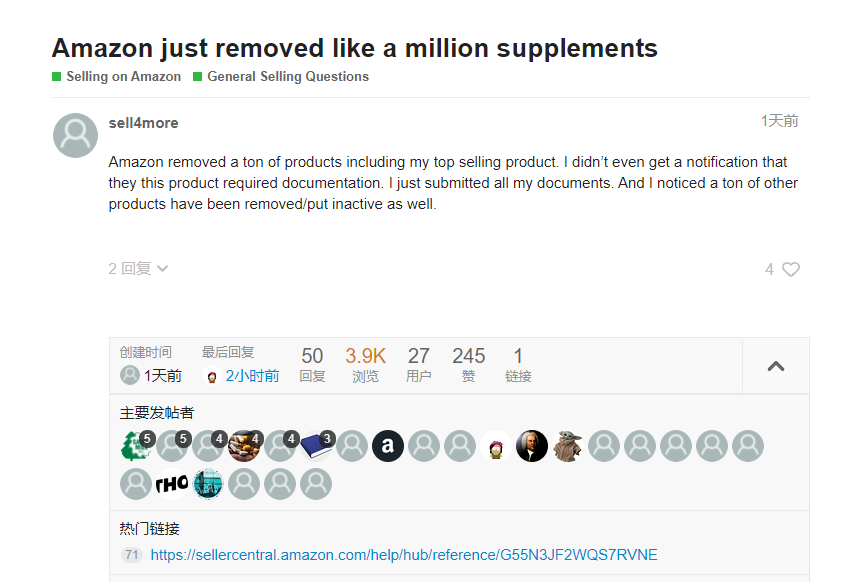
As we shared on November 1, 2021, as part of our continuous efforts to provide the best customer experience, we have implemented additional approval requirements for sex enhancement and weight management supplement products. Starting from January 10, 2022, we are not allowed to publish products that do not meet the requirements of the product documentation
Some sellers believe that strict management of Amazon's products in this category is the right thing to do. Because there are many dietary supplement products on Amazon, and most of these supplements are either lower than the relevant requirements for supplements or have no use at all. Removing these non compliant supplement products is a good thing for consumers.
Some sellers also believe that Amazon's move has caused them some economic losses. Because Amazon directly deleted their product links without prior notice, and some sellers did not restore their product links after submitting compliance documents, this has had a significant negative impact on their operations.
In short, Amazon's decision to strictly manage dietary supplement products is already a matter of certainty. Sellers of this type of purpose must promptly conduct self inspection and upload relevant compliance documents according to Amazon's requirements to avoid any impact on their subsequent operations.